Zirconium metal is one of the most important and irreplaceable metals in the periodic table. Its unique chemical and physical properties make zirconium metal, its alloys, and compounds indispensable in industries such as nuclear energy, biomedicine, and high-temperature materials.
Is Zirconium a Metal?
Yes, zirconium is a metal. Metals are typically characterized by high electrical and thermal conductivity, ductility, malleability, and a lustrous appearance—zirconium meets these criteria as a “transition metal” in the periodic table.
What is Zirconium Metal?
- Atomic number: 40
- Pure zirconium appears silver-gray, while zirconium alloys may exhibit various colors.
- Key properties: Extremely low neutron absorption (ideal for nuclear applications), high melting point, and excellent corrosion resistance, making it suitable for diverse industries.
Common Misconceptions About Zirconium Metal
Myth 1: Zirconium is the same as zircon.
- Clarification: Zircon is a non-metallic compound (zirconium silicate) used in jewelry and thermal barrier coatings. Over 90% of zirconium metal is used exclusively in the nuclear industry, with no overlap in applications. Zircon is inexpensive, while pure zirconium costs ~$30,000 per metric ton.
Myth 2: Zirconium is a rare element.
- Clarification: Zirconium is the 15th most abundant element in the Earth’s crust. Its perceived rarity stems from its use in advanced high-tech industries, making it less familiar to the general public.
Myth 3: Zirconium is as strong as steel.
- Clarification: Steel is significantly stronger than zirconium. While steel is ubiquitous in structural applications, zirconium’s unique properties (not strength) make it suitable for specialized roles like nuclear fuel cladding.
Myth 4: Is zirconium a metal or ceramic?
- Clarification: Zirconium is a metal; zirconia (zirconium oxide) is a ceramic. The similarity in names often causes confusion.
Physical and Chemical Properties of Zirconium
| Property | Value |
|---|---|
| Appearance | Silver-gray, lustrous |
| Density | 6.52 g/cm³ |
| Melting Point | 1855°C |
| Boiling Point | 4409°C |
| Tensile Strength | 330–680 MPa |
| Electrical Conductivity | 2.4 × 10⁶ S/m |
| Thermal Conductivity | 22.7 W/m·K |
| Corrosion Resistance | Excellent |
| Ductility | High |
Key Physical Properties:
- Strength: Moderate strength, suitable for specialized structures (e.g., nuclear fuel cladding, biomedical implants). Alloying elements can enhance strength.
- Density: 6.52 g/cm³ (lower than steel, higher than titanium and aluminum).
- Melting Point: 1855°C, enabling high-temperature applications.
Key Chemical Properties:
- Reactivity: Highly resistant to oxidation but reacts with strong acids and halogens at high temperatures. Reaction with steam at high temperatures caused hydrogen explosions in nuclear plants, a critical safety concern.
- Corrosion Resistance: Excellent in acidic, alkaline, and seawater environments.
- Stability: Maintains integrity in extreme temperatures and harsh chemical conditions.
Comparison with Other Metals
While zirconium may not outperform common metals like titanium, steel, or aluminum in strength or density, its unique properties—corrosion resistance, low neutron absorption, thermal stability, and biocompatibility—make it irreplaceable in specific applications.
Zirconia vs. Titanium:
- Both are biocompatible, corrosion-resistant, and have an HCP crystal structure.
- Applications differ: Zirconium excels in nuclear and high-temperature roles; titanium dominates aerospace and general engineering.
Zirconium vs. Steel:
- Steel has far higher neutron absorption and lacks biocompatibility.
- Zirconium alloys are used in nuclear fuel cladding; steel is unsuitable for such applications.
Production of Zirconium Metal
Different grades of zirconium (e.g., commercial vs. nuclear) are produced based on purity requirements, involving distinct methods and steps.

Extraction Method (Kroll Process):
- Chlorination of Zircon Sand (ZrSiO₄)℃
- Reduction with MagnesiumZrCl4+2MgHigh TempZr+2MgCl2
Refining Process:
Liquid-liquid solvent extraction (using MIBK or TBP) separates zirconium from hafnium (undesirable in nuclear applications due to high neutron absorption).
Challenges and Innovations:
- Key challenges: High cost of hafnium separation, energy-intensive processes, and environmental impacts.
- Innovations: Electrochemical reduction, new purification methods, and recycling to enhance efficiency.
Zirconium Alloys: Types and Applications
Alloying elements are added to zirconium to improve specific properties (e.g., strength for cladding via niobium addition).
| Alloy | Density (g/cm³) | Tensile Strength (MPa) | Corrosion Resistance | Applications |
|---|---|---|---|---|
| Ti Grade 17 (UNS R58640) | 4.64 | 1100–1200 | Excellent at high temps | Aerospace, gas turbines, marine |
| Ti Grade 19 (UNS R58240) | 4.75 | 1150–1250 | Corrosion and wear-resistant | Aerospace, biomedical, structural |
| Zr-2.5Nb | 6.55 | 400–600 | Excellent in acids/high temps | Nuclear reactors, heat exchangers |
| Zr-4, Zr-2 | 6.56 | 500–750 | Superior in nuclear environments | Nuclear fuel cladding, reactors |
Role of Zirconium in Other Alloys:
- Ti-5Al-2Sn-2Zr-4Mo-4Cr (Ti Grade 17): Enhances corrosion resistance, creep resistance, and strength in titanium alloys.
- Ti-3Al-8V-6Cr-4Mo-4Zr (Ti Grade 19): Similar to Ti Grade 17, used in high-strength applications.
Key Zirconium Alloys:
- Zr-2.5Nb: Stabilizes high-temperature performance via niobium, used in nuclear reactors.
- Zr-2/Zr-4: Zr-2 (with nickel) for boiling water reactors; Zr-4 (nickel-free) for pressurized water reactors, optimizing corrosion resistance.
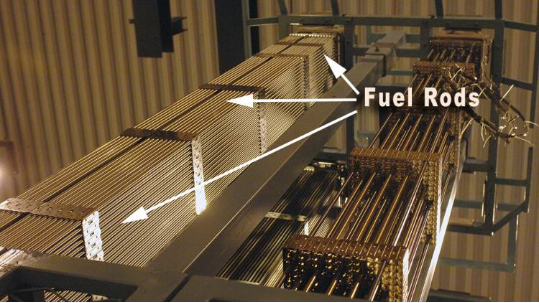
Applications of Zirconium Alloys
- Nuclear Industry: Low neutron absorption makes it ideal for fuel cladding and reactor components.
- Biomedical: Biocompatibility supports use in implants and dental prosthetics.
- Aerospace/High-Temp Environments: Resistance to creep and high temperatures suits turbine components and propulsion systems.
Environmental and Safety Considerations
Safety in Handling:
- Zirconium is non-toxic and biocompatible, but fine zirconium powder poses risks: inhalation may cause respiratory issues, and powder can ignite/spark. Proper ventilation and handling are critical.
Environmental Impact:
- Mining and refining can lead to land degradation, energy waste, and carbon emissions. Recycling reduces resource consumption, though current costs remain high.
Conclusion
Zirconium’s unique properties—particularly its neutron absorption behavior and high-temperature stability—make it irreplaceable in nuclear and specialized industrial applications. While challenges exist in its production, ongoing innovations aim to enhance sustainability and efficiency.
FAQs
- How are zirconium alloys used in medical applications?
- Their biocompatibility and corrosion resistance make them suitable for implants and dental fixtures.
- How does zirconium compare to titanium?
- Titanium is more versatile in general engineering, while zirconium excels in nuclear and high-temperature roles due to unique properties like low neutron absorption.
- Is zirconium metal safe to handle?
- Yes, but zirconium powder requires caution due to potential fire hazards and respiratory risks.