Looking for high-precision, cost-effective manufacturing solutions for medical devices? Injection molding delivers accuracy, regulatory compliance, and scalability for healthcare innovation.
It is a process used to manufacture a wide range of medical equipment—from catheters and syringes to surgical instruments and implant components. This high-precision, efficient method is critical for producing parts that meet the healthcare industry’s strict requirements, including biocompatibility, sterility, and durability.
In this guide, we provide an overview of medical injection molding, discussing material selection, regulatory compliance, applications, and the latest advancements in the field.

What Is Medical Injection Molding?
Medical injection molding is a manufacturing process for producing precision plastic components for the healthcare industry. It enables consistent production of complex, high-volume parts.
The process of manufacturing medical devices via injection molding involves injecting molten medical-grade plastic into a mold, shaping the plastic into parts that meet tight tolerances, are biocompatible, and durable. The molding process typically includes four stages:
- Clamping: The mold is securely closed under high pressure.
- Injection: Molten plastic is injected into the mold cavity.
- Cooling: The material solidifies into the desired shape.
- Ejection: The finished part is removed from the mold.
Medical parts are often molded in controlled environments known as cleanrooms, which minimize the risk of contamination—a critical priority in healthcare. Cleanrooms must adhere to strict standards to produce components that do not compromise patient safety.
All medical device components must comply with rigorous regulatory standards, including ISO 13485 and FDA (Food and Drug Administration) requirements, and undergo detailed quality control processes. These processes include precise documentation, real-time monitoring, and verification at every production stage, ensuring full traceability of materials, processes, and personnel involved.
Why Medical Injection Molding Is Critical in Healthcare
The healthcare industry has unique needs: medical devices must meet strict requirements while remaining cost-effective. Medical injection molding addresses these needs by offering:
- Accuracy: Tight tolerances ensure components are dimensionally precise and assemble as intended.
- Sterility: Cleanroom environments and biocompatible materials prevent contamination, ensuring compliance with FDA and ISO standards.
- Cost-effectiveness: Automated processes and high-volume production reduce costs, helping make healthcare more accessible.
- Durability: Medical-grade plastics withstand repeated sterilization, chemicals, and wear. They also resist mechanical stress and temperature fluctuations.
Common Medical Injection Molding Applications
Below are examples of products manufactured using medical injection molding:
Implantable Devices
Injection molding produces implantable devices such as knee and hip replacements, dental implants, and other surgical implants. These products must be durable and compatible with human tissue.
Surgical Tools
The process is widely used to create high-precision surgical tools, including scalpel handles, forceps, clamps, and retractors. Materials for these tools are selected for strength, biocompatibility, and resistance to repeated sterilization (e.g., autoclaving).
Consumables and Disposable Medical Products
Many single-use medical products are injection-molded, including:
- Syringes
- Test tubes
- Petri dishes
- Drip chambers
Laboratory Equipment
Medical research and testing rely on injection-molded devices such as containers, instrument handles, and microplates.
Medical Device Housings and Enclosures
Many medical devices and testing equipment require enclosures to maintain sterility and protect against environmental factors—often manufactured via injection molding.
Sterile Packaging Solutions
Beyond device manufacturing, injection molding produces medical-grade packaging, such as blister pack components, vials, and containers.

Key Technologies in Medical Injection Molding
Several injection molding techniques are used to manufacture medical parts, including:
- Thin-wall molding
- Gas-assisted molding
- Insert molding
- Overmolding
- Metal injection molding
- Liquid silicone molding
Thin-Wall Molding
Thin-wall injection molding produces lightweight, durable parts with wall thicknesses typically less than 1 mm. It is ideal for small, complex components requiring high precision.
By using less material per part, this method is more cost-effective than traditional injection molding, making it suitable for high-volume production. A common application is creating compact housings for medical devices.
Gas-Assisted Molding
Gas-assisted injection molding is a low-pressure process that combines the precision of traditional injection molding with the benefits of pressurized gas (usually nitrogen or carbon dioxide). The gas pushes molten plastic into every corner of the mold, creating hollow sections in thick-walled parts while reducing material usage. Once the part cools completely, the gas is vented. Minimal post-processing (e.g., trimming flash) is required.
This technique is often used for large-surface-area parts needing detailed textures and superior surface quality, as it achieves smoother finishes, better structural integrity, and fewer visible sink marks. Pressurized gas distributes molten plastic evenly across the mold, eliminating inconsistencies and reducing surface defects.
The gas also maintains uniform pressure during cooling, minimizing internal stress and enhancing structural integrity. By supporting material from the inside, it prevents sink marks, ensuring smooth outer surfaces.
Insert Molding
Insert molding involves molding a secondary component around a preformed insert (often metal, but sometimes plastic or ceramic). The insert is placed in the mold, and molten plastic is injected around it, forming a strong molecular or mechanical bond.
- Molecular bonding occurs when the insert material is the same or similar to the encapsulating resin (e.g., polyurethane Y-connectors molded onto polyurethane catheters), creating a seamless, leak-resistant joint without adhesives or fasteners.
- Mechanical bonding relies on the insert’s surface irregularities (e.g., knurling, flaring, or rough sandblasting) to lock the plastic in place as it cools and shrinks. While shrinkage alone may not ensure a strong bond, mechanical features enhance adhesion—critical for dissimilar materials.
Insert molding is widely used for medical devices such as needle hubs, threaded fasteners, encapsulated electrical components, Luer connectors, and manifolds.
Overmolding
Overmolding involves molding a polymer (e.g., silicone or thermoplastic elastomer [TPE]) onto a substrate to create a single integrated part, eliminating post-assembly steps. Bonds form both chemically (at the molecular level) and mechanically (via physical geometry at the interface of the two molded layers).
Unlike insert molding— which uses preformed (often metal) inserts—overmolding involves molding one material onto another molded part, typically using two different materials in a sequential process. This can be done in a continuous, automated two-shot molding process (ideal for high volumes) or via pick-and-place molding (a manual or semi-automated method suited for prototyping or low volumes). A common application is creating ergonomic handles for surgical tools.
Metal Injection Molding (MIM)
Metal injection molding (MIM) starts with a mixture of metal powder and thermoplastic binder. The mixture is heated, injected into a mold, and cooled to form a part. After ejection, the binder is removed, and the part is sintered—a high-temperature process that fuses metal particles into a strong, dense, precise component.
MIM is often used for surgical instruments and implants, excelling at high-volume production of small, complex medical parts. It offers superior strength, excellent surface finishes, and tight tolerances, serving as a cost-effective alternative to CNC machining—especially for hard-to-machine metals like stainless steel, cobalt-chromium, and titanium alloys.
Liquid Silicone Molding
Liquid silicone molding produces medical products such as respiratory masks, tubing, and implants. Silicone is inherently biocompatible, making it ideal for medical applications.
The process uses liquid silicone rubber (LSR), a two-part elastomer that cures via chemical cross-linking. In liquid silicone injection molding, the mixture is injected into the mold under high pressure and cures in approximately 30 seconds. A controlled, sterile environment is required to prevent contamination from dust, moisture, or airborne particles, which could compromise the final product.
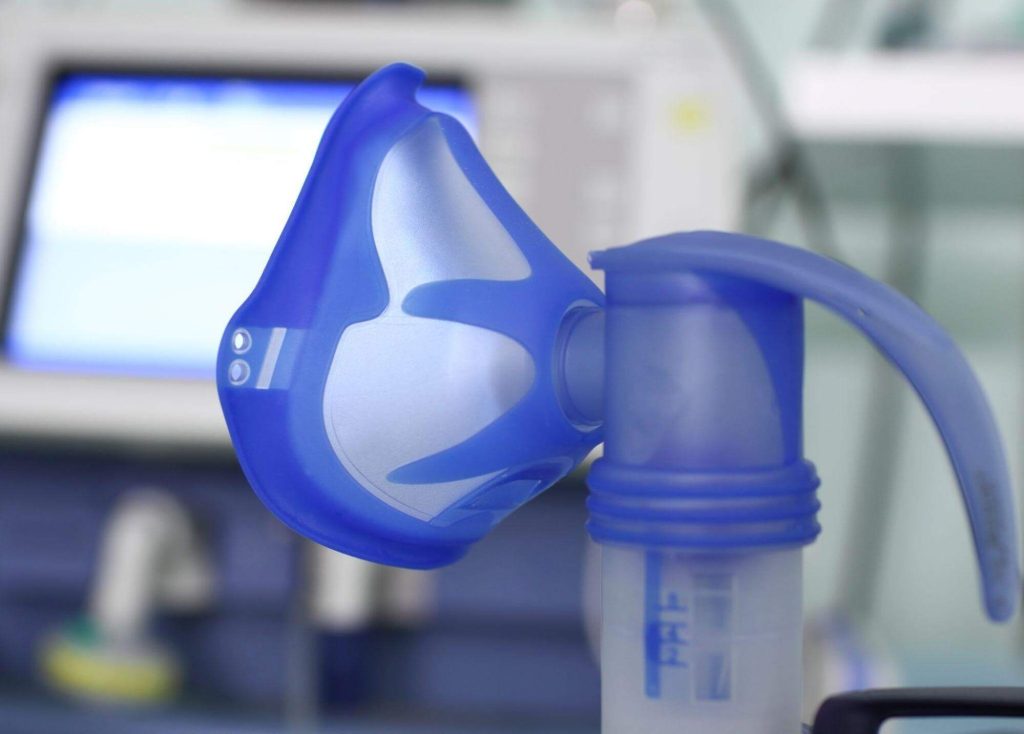
Plastic Materials for Medical Injection Molding
Injection molding uses various materials, including thermoplastics and thermosets. However, medical devices primarily use thermoplastic polymers, which can be heated, molded, and remelted repeatedly without structural degradation—ideal for medical applications.
Thermoplastics are melted, injected into molds, cooled, and solidified into the desired shape. Mold designs range from simple two-plate tools to complex configurations, depending on part requirements.
Thermosets, by contrast, are unsuitable for most medical injection molding. They form strong covalent bonds during curing and cannot be remelted or reshaped, limiting recyclability and adaptability for medical needs. They are more commonly used in adhesives, protective coatings, or high-temperature applications.
Common medical-grade plastics for injection molding include:
Polyethylene (PE)
This medical-grade plastic offers impressive rigidity and tensile strength, withstands harsh environments, and is highly compatible with human tissue. Applications include tubing, connectors, prosthetics, and containers. Types of PE include:
- High-density polyethylene (HDPE): Rigid and moisture-resistant, used for medication bottles, prescription vials, syringe components, medical packaging, lab jars, and ointment tubes.
- Low-density polyethylene (LDPE): Translucent and flexible, used for squeezable bottles and medical tubing.
- Ultra-high-molecular-weight polyethylene (UHMW): Exceptionally wear-resistant, ideal for orthopedic implants.
Polypropylene (PP)
PP offers superior strength, better moisture and gas resistance than PE, and is impact-resistant, crack-resistant, and resistant to temperature degradation, wear, and radiation. It is suitable for storing liquid medications and is used in critical components like knee/hip replacements (as liners and spacers to minimize metal-on-metal contact), syringes, and connectors.
Polycarbonate (PC)
A naturally transparent material with excellent mechanical properties—flexibility, toughness, impact resistance, and compatibility with biological tissues. It is used in protective gear, transparent masks, and oxygenators.
Polyetheretherketone (PEEK)
PEEK resists harsh environments, including high temperatures, chemicals, radiation, and wear. This high-performance thermoplastic maintains dimensional stability even under stress, making it ideal for orthopedic implants and prosthetics.
Polystyrene (PS)
While less flexible than other materials, PS offers excellent mechanical properties, biocompatibility, and dimensional stability. It is used in lab trays, petri dishes, gloves, vials, pipettes, implants, and diagnostic components.
Perfluoroalkoxy (PFA)
A fluoropolymer with high heat resistance, excellent creep resistance at high temperatures, and chemical resistance.
Fluorinated Ethylene Propylene (FEP)
Another fluoropolymer with low friction, good impact resistance, chemical resistance, flexibility, transparency, and biocompatibility.
Thermoplastic Elastomers (TPEs)
TPEs balance thermoplastic processability with rubber-like elasticity, making them ideal for overmolded components or catheters.
Silicone
Though not a plastic, silicone is a rubber-like material with inherent biocompatibility, used in tubing, connectors, and catheters.

Standards and Requirements for Medical Injection Molding
Medical plastic molding is a highly precise, critical process with little room for error. Selected materials must meet strict requirements to ensure safety, functionality, and reliability. Key considerations include:
Regulatory Compliance
Manufacturing injection-molded medical parts requires adherence to rigorous standards. The healthcare industry is heavily regulated, and components must meet strict FDA requirements and ISO certifications to ensure safety, efficacy, and reliability for patient use.
FDA Regulations
The FDA enforces strict standards for cleanliness, sterility, and overall safety of medical devices, covering implants, surgical instruments, and other components. Key considerations include:
- Material suitability: Materials must withstand sterilization (autoclaving, gamma radiation, or ethylene oxide) without degradation and be biocompatible (no adverse reactions when in contact with human tissue or fluids).
- Manufacturing processes: Injection molding must comply with FDA guidelines, including audits to ensure facilities follow Good Manufacturing Practices (GMP) and maintain cleanrooms to prevent contamination.
- Documentation and traceability: Manufacturers must retain detailed records of materials, processes, and quality control measures to demonstrate compliance during FDA inspections.
Non-compliance can lead to product recalls, legal penalties, and reputational damage. Thus, partnering with material and manufacturing suppliers with a strong FDA compliance track record is critical.
Medical Device Classification
Medical devices are classified based on the risk of harm to patients, the public, or users. This classification determines the level of regulatory oversight:
- Class I: Low-risk devices not intended to sustain life or pose unreasonable risk (e.g., wheelchairs, eyeglasses, bedpans, test tubes).
- Class II: Devices where general controls alone are insufficient to ensure safety/effectiveness (e.g., contact lenses, syringes, pregnancy tests). The EU MDR further divides Class II into IIa (medium risk) and IIb (high risk).
- Class III: High-risk devices, often life-sustaining, with potential for significant harm (e.g., pacemakers, defibrillators, implantable prosthetics).

ISO Certifications
The medical device industry is tightly controlled to ensure patient safety. Key standards affecting manufacturing (including plastic injection molding) include:
- ISO 13485 – Medical Devices: Outlines requirements for quality management systems (QMS) in medical product manufacturing. It applies to all manufacturing technologies, ensuring consistent quality and safe, effective production. It defines best practices for documentation, training, traceability, design, production, and non-conformity management. In the U.S., it is often supplemented or replaced by FDA’s Quality System Regulation (QSR, 21 CFR Part 820), which shares many similarities.
- ISO 10993 – Biological Evaluation of Medical Devices: Focuses on biocompatibility requirements for devices in direct or indirect contact with the body. It aims to minimize adverse patient reactions to materials (including injection-molded plastics), ensuring they are non-toxic, non-immunogenic, and non-carcinogenic. Comprising 23 parts, it covers animal welfare, carcinogenicity, reproductive toxicity, in vitro cytotoxicity, and sterilization. USP Class VI (U.S. Pharmacopeia) covers similar biocompatibility requirements and is sometimes used alongside or instead of ISO 10993.
- ISO 14644 – Cleanrooms and Associated Controlled Environments: Standardizes cleanroom use in medical device manufacturing, defining classes 1–9 (Class 1 being the strictest). Cleanrooms control air particle count and size to reduce contamination risks. Designers may require Class 7 or 8 cleanrooms for injection molding to limit manufacturing-related pollution.

Sterilization Resistance
Sterilization is mandatory for medical devices. Components in contact with the body must resist contamination and withstand sterilization methods (autoclaving, gamma radiation, chemical treatments) without damage.
Operating Environment
Medical devices often function in challenging conditions (high temperatures, chemicals, liquids, vibration, physical stress). Materials must be reliable and durable, maintaining performance under such stress.
Durability and Strength
Fragile materials are unacceptable in healthcare—especially for implants and surgical tools. Selected materials must have high durability and tensile strength to minimize biohazard risks.
Medical Injection Molding in Cleanrooms
Latest Advancements and Future Innovations in Medical Injection Molding
Driven by cutting-edge technology and emerging trends, medical injection molding is evolving rapidly, transforming the design and manufacturing of medical devices. These advancements improve precision, efficiency, and enable more complex, customized, and sustainable solutions. Key innovations include:
Scientific Molding and AI Optimization
The demand for high-precision, consistent medical devices has led to the development of scientific molding—a data-driven approach to optimizing injection processes. Real-time monitoring and advanced analytics ensure each part meets exact specifications.
A major advancement is the integration of artificial intelligence (AI) into molding processes. AI algorithms continuously adjust parameters (temperature, pressure, cycle time) to enhance quality and reduce material waste, improving efficiency and supporting sustainability.
Integration with 3D Printing
3D printing is revolutionizing prototyping and production of medical devices. By combining 3D printing with traditional injection molding, manufacturers accelerate prototyping and create highly customized components.
For example, 3D-printed injection molds enable testing new designs before investing in expensive tooling, reducing development time and costs. This hybrid approach is particularly valuable for complex geometries previously unachievable with traditional methods and for meeting patient-specific needs in healthcare.
Advanced Biocompatible Materials
Material science innovations are yielding new polymers with enhanced properties (strength, flexibility, heat resistance) and biocompatibility. These materials expand design possibilities for implants and minimally invasive surgical tools.
Sustainability and Eco-Friendly Practices
Efforts are underway to make the medical injection molding industry more environmentally sustainable. Research focuses on compostable or recyclable materials, while energy-efficient equipment and processes minimize waste and reduce carbon footprints.
For more information, please contact Debaolong Seiko. You are also welcome to upload your design to Debaolong Seiko for a quote.